 Researchers from the Norwegian University of Science and Technology (NTNU) are working on improving the solid electrolyte interphase (SEI) as a way to boost battery life.
Researchers from the Norwegian University of Science and Technology (NTNU) are working on improving the solid electrolyte interphase (SEI) as a way to boost battery life. Researchers from the Norwegian University of Science and Technology (NTNU) are working on improving the solid electrolyte interphase (SEI) as a way to boost battery life.
Researchers from the Norwegian University of Science and Technology (NTNU) are working on improving the solid electrolyte interphase (SEI) as a way to boost battery life.
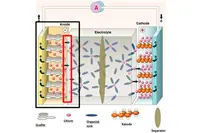
When a lithium battery is first charged up, a thin SEI film forms on the surface of the carbon anode. This film has a complex chemical structure containing both organic and inorganic lithium compounds. This protective coating is key in determining a battery’s lifetime, thermal stability and capacity, especially at high rates.
Lithium that is bound in the film doesn’t participate in charging the electrodes which results in reduced capacity. Ahmet Oguz Tezel, a PhD candidate at NTNU’s Department of Materials Science and Engineering, said: “Once the battery is assembled, we cannot add more lithium to the cell, therefore limiting the loss of available lithium in the cell is of prime importance for long lasting batteries.”
Tezel’s work has focused on modifying the composition of the electrolyte to achieve higher battery capacity and life span, especially at low temperatures.
He has also had promising results with his work with developing a preparatory treatment that prevents too much lithium loss in the formation of the SEI film. This allows more of the lithium in the electrolyte solution to participate in charging the electrodes.
State of the art electrolytes that consist of ethylene carbonate (EC), in principle, cannot operate when the temperature is at about -10°C, due to the high melting temperature of the EC. On the other hand batteries containing the organic compound propylene carbonate (PC) can work at temperatures as low as -50°C.
“We think that we found a way to substitute EC for PC, however we haven’t confirmed this yet,” Tezel said. “But our research suggests how it might be realised.”
Pic: Lithium in the highlighted layer cannot be used for charging or powering the battery. The goal is to limit the amount of lithium that is bound up in this layer. Key: grafitt = graphite; litium = lithium; organisk syre = organic acid; katode = cathode
Author
Tom Austin-Morgan
Source: www.newelectronics.co.uk