Superoxide gives lithium-air batteries a jolt
 Researchers at the US Department of Energy’s Argonne National Laboratory are currently trying to find chemistries that could offer better energy possibilities. The researchers say that, of these chemistries, lithium-air could promise greater energy density.
Researchers at the US Department of Energy’s Argonne National Laboratory are currently trying to find chemistries that could offer better energy possibilities. The researchers say that, of these chemistries, lithium-air could promise greater energy density.
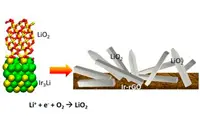
Previous work on lithium-air batteries has shown the same phenomenon: the formation of lithium peroxide, a solid precipitate that clogged the pores of the electrode.
In a recent experiment, however, the Argonne battery scientists were able to produce stable crystallised lithium superoxide instead of lithium peroxide during battery discharging. Unlike lithium peroxide, lithium superoxide can dissociate into lithium and oxygen, leading to high efficiency and good cycle life.
"This discovery really opens a pathway for the potential development of a new kind of battery," Larry Curtiss, one of the scientists, said. "Although a lot more research is needed, the cycle life of the battery is what we were looking for."
The major advantage of a battery based on lithium superoxide, Curtiss explained, is that it theoretically allows for the creation of a lithium-air battery that consists of what chemists call a ‘closed system’. Open systems require the consistent intake of extra oxygen from the environment, while closed systems do not - making them safer and more efficient.
Khalil Amine, Curtiss’ colleague, explained: "The stabilisation of the superoxide phase could lead to developing a new closed battery system based on lithium superoxide, which has the potential of offering truly five times the energy density of lithium ion."
The scientists attributed the growth of the lithium superoxide to the spacing of iridium atoms in the electrode used in the experiment. "It looks like iridium will serve as a good template for the growth of superoxide," Curtiss said.
However, the scientists admit that they have to learn how to design catalysts to understand exactly what's involved in lithium-air batteries.
Author
Tom Austin-Morgan
Source: www.newelectronics.co.uk
 Researchers at the US Department of Energy’s Argonne National Laboratory are currently trying to find chemistries that could offer better energy possibilities. The researchers say that, of these chemistries, lithium-air could promise greater energy density.
Researchers at the US Department of Energy’s Argonne National Laboratory are currently trying to find chemistries that could offer better energy possibilities. The researchers say that, of these chemistries, lithium-air could promise greater energy density.