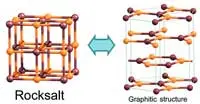
 An international team of researchers has used computer simulations to find how thin a slab of salt has to be for it to break up into graphene-like layers.
An international team of researchers has used computer simulations to find how thin a slab of salt has to be for it to break up into graphene-like layers. An international team of researchers has used computer simulations to find how thin a slab of salt has to be for it to break up into graphene-like layers.
An international team of researchers has used computer simulations to find how thin a slab of salt has to be for it to break up into graphene-like layers.
Dr Pavel Sorokin, from Moscow’s Technological Institute for Superhard and Novel Carbon Materials, explained: “This work has already attracted our colleagues from Israel and Japan. If they confirm our findings experimentally, this phenomenon [of graphitisation] will provide a viable route to the synthesis of ultrathin films with potential applications in nanoelectronics.”
Previous theoretical studies suggested that films with a cubic structure and ionic bonding could spontaneously convert to a layered hexagonal graphitic structure. However, no theory had been developed that would account for this process in the case of an arbitrary cubic compound and make predictions about its conversion into graphene-like salt layers.
To study how graphitisation tendencies vary depending on the compound, the researchers examined 16 binary compounds. They all had a hexagonal, ‘graphitic’ phase that is unstable in 3D bulk but becomes the most stable structure for ultrathin 2D films. The researchers identified the relationship between the surface energy of a film and the number of layers in it for both cubic and hexagonal structures. They graphed this relationship by plotting two lines with different slopes for each of the compounds studied. Each pair of lines associated with one compound had a common point that corresponded to the critical slab thickness that makes conversion from a cubic to a hexagonal structure energetically favourable. The critical number of layers was found to be close to 11 for all sodium salts and between 19 and 27 for lithium salts.
Based on this data, the researchers established a relationship between the critical number of layers and two parameters that determine the strength of the ionic bonds in various compounds: the ionic radius and electronegativity. Higher electronegativity means more powerful attraction of electrons by the atom, a more pronounced ionic nature of the bond, a larger surface dipole, and a lower critical slab thickness.
Author
Peggy Lee
Source: www.newelectronics.co.uk