 Scientists at Stanford University and the US Department of Energy’s SLAC National Accelerator Laboratory have discovered a way to use diamondoids – the smallest possible bits of diamond – to assemble atoms into electrical wires just three atoms wide.
Scientists at Stanford University and the US Department of Energy’s SLAC National Accelerator Laboratory have discovered a way to use diamondoids – the smallest possible bits of diamond – to assemble atoms into electrical wires just three atoms wide.
The technique could potentially be used to build wires for a range of applications, including fabrics that generate electricity, optoelectronic devices that employ both electricity and light, and superconducting materials that conduct electricity without loss.
“What we have shown here is that we can make tiny, conductive wires that essentially assemble themselves,” said Hao Yan, a Stanford postdoctoral researcher. “The process is a simple, one-pot synthesis. You dump the ingredients together and you can get results in half an hour.”
According to the researchers, although there are other ways to get materials to self-assemble, this is the first one shown to make a nanowire with a solid, crystalline core that has good electronic properties.
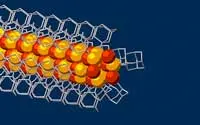
For this study, the research team started with the smallest possible diamondoids – single cages that contain 10 carbon atoms – and attached a sulphur atom to each. Floating in a solution, each sulphur atom bonded with a single copper ion. This created the basic nanowire building block.
“Much like LEGO blocks, they only fit together in certain ways that are determined by their size and shape,” said Stanford graduate student Fei Hua Li. “The copper and sulphur atoms of each building block wound up in the middle, forming the conductive core of the wire, and the bulkier diamondoids wound up on the outside, forming the insulating shell.”
The team has already used diamondoids to make one-dimensional nanowires based on cadmium, zinc, iron and silver.
“You can imagine weaving them into fabrics to generate energy,” said Nicholas Melosh, an associate professor at SLAC and Stanford. “This method gives us a versatile toolkit where we can tinker with a number of ingredients and experimental conditions to create new materials with finely tuned electronic properties and interesting physics.”
Author
Peggy Lee
Source: www.newelectronics.co.uk
